
A prominent Australian oncology researcher says ovarian cancer patients are losing out as badly-needed funds and clinical attention is focused on more prominent – yet not as deadly – women’s diseases.
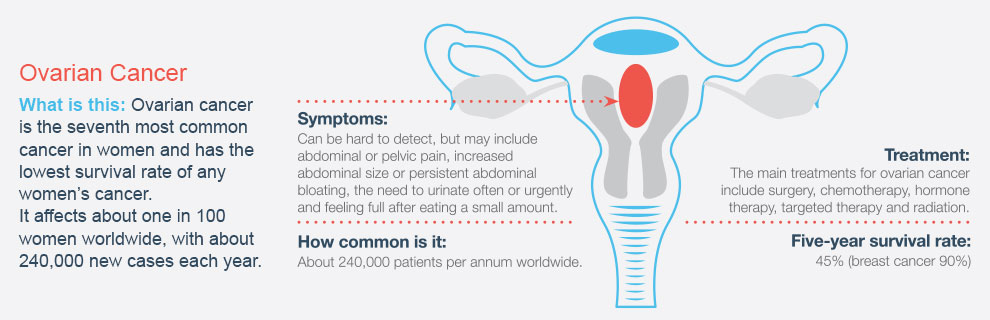
Tomorrow (8 May) is World Ovarian Cancer Day. Ovarian cancer is the eighth most common cancer in women, and the sixth most common cause of cancer death for women in Australia.1 The Women’s Cancer Foundation states that only 20% of the 1400 Australian women diagnosed with ovarian cancer every year will survive for longer than five years, compared to breast cancer which has an 80% survival rate.2
Associate Professor Jim Coward, an oncologist who is leading the first clinical trial of the Australian therapy Cantrixil for recurrent ovarian cancer, said ovarian cancer treatment was far behind that of the more publicised breast and cervical cancers.
“More needs to be done in developing novel clinical trials to help enhance the survival rates for women with ovarian cancer,” Dr Coward said.
“We’re really only gaining small steps in understanding ovarian cancer – how it evolves, how the tumour behaves once its established, where it originates – and making small gains subsequently in developing effective treatments.
“Many women wrongly assume that when they’re getting their Pap test, they’re also getting screened for ovarian cancer. While there are a couple of ways doctors currently test for ovarian cancer, these processes are controversial.
“Ovarian cancer presents late and in late stages. If we could be on the front foot with it, treatments are likely to be more effective.
“Ovarian cancer needs a greater national focus and a unified approach to screening.”
Dr Coward completed his medical training at Imperial College, London in 1998 and became a member of the Royal College of Physicians in 2002. His interests in oncology began during his tenure as a junior doctor and he subsequently undertook specialist registrar training at Westmead Hospital, Sydney and the Royal Marsden Hospital, London.
In 2006, he was appointed as a MRC clinical fellow at the Barts Cancer Institute, London and investigated the effects of the anti-IL-6 class of therapeutics in advanced ovarian cancer which saw the transition of laboratory research into a clinical trial in patients with platinum resistant (or persistent) disease. Dr Coward has a PhD in cancer immunotherapy.
Cantrixil is being developed by the Australian biotech Kazia Therapeutics (ASX: KZA; NASDAQ: KZIA) and could enable intraperitoneal (IP) chemotherapy to again become a viable treatment option in ovarian cancer patients where it has become ineffective due to resistance.3
More about Cantrixil: https://www.kaziatherapeutics.com/researchpipeline/cantrixil
1 Cancer Council Australia [online] http://www.cancer.org.au/about-cancer/types-of-cancer/ovarian-cancer.html [accessed] 17 August 2017.
2 Women’s Cancer Foundation [online] http://www.womenscancerfoundation.org.au/pages/research/statistics [accessed 3 May 2018].
3 Wasif Saif M, Heaton A, Lilischkis K, Garner J, Brown D.M, Pharmacology and toxicology of the novel investigational agent Cantrixil (TRX-E-002-1), Journal of Cancer Chemotherapy and Pharmacology, 2016, DOI 10.1007/s00280-016-3224-2 [ONLINE] https://nrt.irmau.com/irm/PDF/1583_0/JournalofCancerChemotherapyandPharmacology
***END***
About Kazia Therapeutics
Kazia Therapeutics Limited (ASX: KZA, NASDAQ: KZIA) is an innovative oncology-focused biotechnology company, based in Sydney, Australia. Our pipeline includes two clinical-stage drug development candidates, and we are working to develop therapies across a range of oncology indications.

